Methoxypropyl Acetate Definition
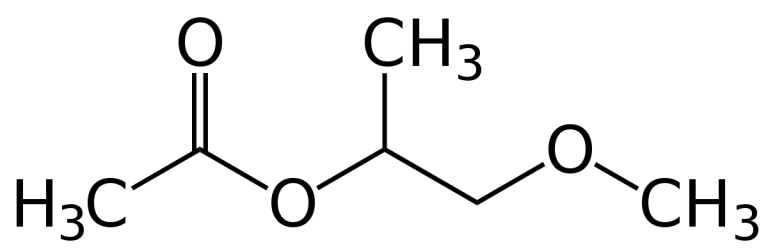
Methoxypropyl Acetate, also known as propylene glycol monomethyl ether acetate, is a clear, colourless liquid with a mild ether-like odour. It is only slightly soluble in water but miscible with most common organic solvents. Methoxy propyl acetate is slightly hygroscopic, relatively fast evaporating and has a low viscosity. It enters into reactions which are characteristic of both esters and ethers, displaying their good solvent power. Methoxy propyl acetate is produced by reacting propylene oxide with methanol using a catalyst. it is obtained in the reaction of the esterification with the acetic acid of glycol monomers obtained in the reaction of the ethylene oxide or propylene with alcohol.
Methoxypropyl acetate Is also known as 1-methoxypropyl acetate; Methoxypropanol acetate; Lunazard; PM Acetate; Bawanol PMA
Methoxypropyl Acetate Applications
By virtue of its good solvent power for numerous resins and dyes, Methoxypropyl Acetate can be used as a solvent, flow improver and coalescent in coatings.
It is particularly suitable for coatings containing polyisocyanates. In this case, it is important that the peroxide fraction is as small as possible, as otherwise the colour of the final product may change. It is for this reason that we stabilize our solvent with 2,6-di-t-butyl-p-cresol (BHT).
- Main application of methoxy propyl acetate are in coatings and printing inks, where it is frequently used as an alternative to ethoxyethyl acetate.
- Used as a solvent
- Suitable for coatings containing polyisocyanates
- Flexographic, gravure and screen printing inks
- Adhesives
- Ball pen pastes
- Dyes in furniture polishes or wood stains
- Dye solutions and pastes for printing and colouring leather and textiles.
Methoxypropyl Acetate CHARACTERISTICS
Methoxypropyl Acetate is a clear, slightly hygroscopic liquid with a mild odour. It is freely miscible with most common organic solvents, but has only limited miscibility with water.
By virtue of its ether and ester groups, Lunazard enters into reactions that are characteristic of ethers and esters and display their solvent power. For instance, it dissolves numerous natural and synthetic resins, waxes, fats and oils.
Since Lunazard may react with the oxygen in the air to form peroxides, BASF supplies it inhibited with 2.6-di-tert-butyl-para-cresol (butylated hydroxytoluene – BHT).
- clear
- slightly hygroscopic liquid
- mild odour
- miscible with most common organic solvents
- limited miscibility with water
| CHARACTERISTIC | Condition | Value | Test method |
| Boiling range | at 1013 hPa; 95
Vol.-%; 2 – 97 ml |
145 – 147°C | DIN 53171 |
| Density | 20°C | 0.965 – 0.970
g/cm3 |
DIN 51757 |
| Refractive index n20D | 1.401 – 1.403 | DIN 51423 | |
| Solidification point | Below -75°C | ||
| Evaporation rate | ether = 1 | 33 | DIN 53170 |
| Enthalpy of combustion (D Hc)
|
at 25°C | 24 208 kJ/kg | |
| Enthalpy of formation(D Hf) | at 25°C | -5 036 kJ/kg | |
| Enthalpy of vaporization(D Hv) | at 25°C | 380.6 kJ/kg | |
| Enthalpy of vaporization(D Hv) | at boiling point | 317.3 kJ/kg | |
| Surface tension o | at 20°C | 28.2 mN/m | |
| Surface tension o | at 40°C | 25.2 mN/m | |
| Solubility | at room temperature | ||
| Methoxypropyl Acetate in water | Approx. 22% wt |
Storage & Handling
Methoxypropyl Acetate should be stored under nitrogen. The storage temperature must not exceed 40°C and moisture are excluded. Under these conditions, a storage stability of 12 months can be expected.
Safety
When using this product, the information and advice given in our Safety Data Sheet should be observed. Due attention should also be given to the precautions necessary for handling chemicals.
